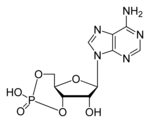
Cyclic adenosine monophosphate (cAMP, cyclic AMP or 3'-5'-cyclic adenosine monophosphate) is a second messenger important in many biological processes. cAMP is derived from adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway.
Synthesis and decomposition
cAMP is synthesised from ATP by adenylyl cyclase located at the cell membranes. Adenylyl cyclase is activated by a range of signaling molecules through the activation of adenylyl cyclase stimulatory G (Gs)-coupled receptors and inhibited by agonists of adenylyl cyclase inhibitory G (Gi)-protein-coupled receptors. Liver adenylyl cyclase responds more strongly to glucagon, and muscle adenylyl cyclase responds more strongly to adrenaline.
cAMP decomposition into AMP is catalyzed by the enzyme phosphodiesterase.
[edit] Functions
cAMP is a second messenger, used for intracellular signal transduction, such as transferring the effects of hormones like glucagon and adrenaline, which cannot pass through the cell membrane. It is involved in the activation of protein kinases and regulates the effects of adrenaline and glucagon. It also regulates the passage of Ca2+ through ion channels.
[edit] In humans
Main article: function of cAMP-dependent protein kinase
Epinephrine (adrenaline) binds its receptor, which associates with an heterotrimeric G protein. The G protein associates with adenylyl cyclase, which converts ATP to cAMP, spreading the signal (more details...)
cAMP and its associated kinases function in several biochemical processes, including the regulation of glycogen, sugar, and lipid metabolism.
In humans, cyclic AMP works by activating protein kinase A (PKA, cAMP-dependent protein kinase). PKA is normally inactive as a tetrameric holoenzyme, consisting of two catalytic and two regulatory units (C2R2), with the regulatory units blocking the catalytic centers of the catalytic units. Cyclic AMP binds to specific locations on the regulatory units of the protein kinase, and causes dissociation between the regulatory and catalytic subunits, thus activating the catalytic units and enabling them to phosphorylate substrate proteins.
The active subunits catalyze the transfer of phosphate from ATP to specific serine or threonine residues of protein substrates. The phosphorylated proteins may act directly on the cell's ion channels, or may become activated or inhibited enzymes. Protein kinase A can also phosphorylate specific proteins that bind to promoter regions of DNA, causing increased expression of specific genes. Not all protein kinases respond to cAMP. Several classes of protein kinases, including protein kinase C, are not cAMP-dependent.
Further effects mainly depend on cAMP-dependent protein kinase, which vary based on the type of cell.
Still, there are some minor PKA-independent functions of cAMP, e.g., activation of calcium channels, providing a minor pathway by which growth hormone-releasing hormone causes a release of growth hormone.[1]
[edit] In non-humans
[edit] Role of cAMP in bacteria
In bacteria, the level of cAMP varies depending on the medium used for growth. In particular, cAMP is low when glucose is the carbon source. This occurs through inhibition of the cAMP-producing enzyme, adenylyl cyclase, as a side-effect of glucose transport into the cell. The transcription factor cAMP receptor protein (CRP) also called CAP (catabolite gene activator protein) forms a complex with cAMP and thereby is activated to bind to DNA. CRP-cAMP increases expression of a large number of genes, including some encoding enzymes that can supply energy independent of glucose.
cAMP, for example, is involved in the positive regulation of the lac operon. In an environment of a low glucose concentration, cAMP accumulates and binds to the allosteric site on CRP (cAMP receptor protein), a transcription activator protein. The protein assumes its active shape and binds to a specific site upstream of the lac promoter, making it easier for RNA polymerase to bind to the adjacent promoter to start transcription of the lac operon, increasing the rate of lac operon transcription. With a high glucose concentration, the cAMP concentration decreases, and the CRP disengages from the lac operon
Regulation of the nuclear import of many transcription factors represents a step in gene regulation which is crucial for a number of cellular processes. The aryl hydrocarbon receptor (AHR), a basic helix-loop-helix protein of the PAS (PER-ARNT-SIM) family of transcriptional regulators is a cytosol-associated and ligand-activated receptor. The environmental toxin dioxin binds with high affinity to AHR rendering it nuclear and leading to the activation of AHR sensitive genes. However, the fact, that the AHR mediates a large variety of physiological events without the involvement of any known exogenous ligand, including liver and vascular system development, maturation of the immune system, regulation of genes involved in cellular growth, cell differentiation and circadian rhythm, speaks for an important role of AHR in cell biology independent of the presence of an exogenous ligand. Different approaches were applied to study mechanism(s) which render AHR nuclear and design its function in absence of exogenous ligands. We found that AHR is sensitive to cAMP signaling mediated by cAMP-dependent protein kinase (PKA) which fundamentally differs from AHR signaling mediated by the exogenous ligand dioxin. It has been shown that PKA mediated signaling can be confined by compartmentalization of signaling components in microdomains conferring specificity to signaling by the ubiquitous second messenger cAMP. Moreover, A-kinase-anchoring proteins (AKAPs) and newly discovered cAMP receptors, Epac (exchange protein directly activated by cAMP), may give us a further chance to enter into new dimensions of cAMP signal transmissions that potentially may bring us closer to AHR physiology.
No comments:
Post a Comment